
Hygienic behavior of different bees species in response to Varroa mites
Hygienic behavior of honey bees reflects social immunity to parasites and diseases and is considered one of the main factors for genetic resistance in honey bee breeding programs. Hygienic behavior refers to the removal and removal of diseased and dead larvae and pupae from sealed pupae in honeycombs by worker bees. This study investigated the effects of needle killing and artificial infestation of larval hives with mites on the hygienic behavior of Apis mellifera (A. melanogaster) species. In addition, reproductive behavior of Varroa mites was determined in hives of different sizes. The results showed that the percentage of decapping and removal of dead bees was significantly higher in Italian honeycombs compared with that in Carniola honeycombs (p < 0.05). Similarly, there were significant differences in the responses of Italian honeycombs and Carniola honeycombs to artificially infested pupae with Varroa mites on the day of examination (p < 0.05). Overall, there were significant differences in the cell apertures of the two honeycombs. The smaller the width of the cell aperture, the more Varroa mites were reduced in reproductive behavior. Drone honeycomb pupae had more Varroa infestations compared with worker honeycombs in both new and old honeycombs. Given the important contribution of honey bee hygienic behavior to honey bee health, this study helps to understand the hygienic behavior of western honey bees and prepare for the selection of hygienic honey bee strains.

1. Introduction
The major ectoparasitic mites of honey bees are Varroa destructans, Andersen and Truman ( Acari : Varroidae) which cause serious damage to honey bee health all over the world. It is a major pest of Asian Oriental honey bees (Apis cerana) but is currently consider a serious threat to Western honey bees (A. mellifera). Infestations of mites have caused a large number of Western honey bee colony losses in many countries over the past few decades. These mites directly feed on the adipose tissue/hive components of immature and mature bees and indirectly transmit various deadly honey bee viruses and bacteria, thereby causing serious damage to honey bees.
Mite infestations can cause shortened lifespan, deformities, weight loss and colony weakness in the hosts. Since the beginning of the 19th century, various techniques such as mechanical, chemical and natural procedures have been develop to combat Varroa mites to prevent colony losses. Among them, chemical methods are effectively use to control the infestation of mites, but it produces resistance of the mites to most acaricides and even induces contaminated hive products. Different alternative control strategies such as physical removal of mites and natural substances such as essential oils and plant extracts have been adopt to minimize the infestation of mites. One of the most promising approaches to reducing Varroa infestations is through artificial selection of mite-resistant bees, such as the Varroa-sensitive hygienic (VSH) and Minnesota hygienic (HYG) strains. VSH and HYG colonies have higher hygienic practices and are consider more resistant to Varroa mites than unselected colonies.
Naturally, social insects such as honey bees, wasps and white wasps have developed different behavioral habits to prevent the spread of pathogens and diseases in the colony. Specifically, the hygienic behavior of honey bees has been studied for nearly 80 years to understand the mechanisms that reduce pathogen infestation and mite resistance and improve the health of their colonies. Rothenbuhler (1964) introduced the term “hygienic behavior” to describe the ability of worker bees to detect, remove and remove diseased and dead bee species from the hive in order to reduce infection. The hygienic behavior of honey bees is measure using two methods: the needle prick kill test (PKB) and the freeze kill test (FKB). It is a successful defense mechanism against diseases of honey bees, such as American brood disease, chalkbrood and Varroa destructor.
In honey bees, hygienic behavior is a heritable genetic trait control by two to seven loci. Some species of honey bees have the ability to minimize mite infestation levels in worker cells, significantly reducing the reproductive success of mites.
Therefore, colonies with higher hygienic behavior are suggest as a natural strategy to control pathogens, pests, and disease infestations. In general, the environment and genotype are the factors that determine the hygienic behavior of honey bees. In this study, we conducted different experiments with the following objectives:
(1) To evaluate the hygienic behavior of populations of Apis mellifera ligustica and Apis carnivora towards uncapped and nailed larvae in capped larval chambers;
(2) evaluate the hygienic behavior of two honey bee populations in larval chambers artificially infested with Varroa mites;
(3) Determine the reproductive behavior of Varroa mites of different cell sizes on the nest foundation;
(4) Compare the infection rate of Varroa mites in new and old hives. This study provides a new way for beekeepers to control Varroa mites, which is beneficial to increase honey production and improve the economic conditions of beekeepers.
2. Materials and Methods
2.1. The hygienic behavior of honey bees was investigate using the needle killing assay. Eight colonies were divide into two groups based on queens, one with Apis mellifera and the other with Honey bees Carniola. Both complete colonies were in hives of Langstroth. All colonies had viable queens , workers, larvae, large quantities of honey and pollen.
To determine the hygienic behavior, larval cell perforation (needle killing assay) was apply using the method describe by Büchler et al. (2013) and Newton and Ostasiewski Jr (1986). Briefly, the area with the most sealed larval cells was select, 100 brood worker larvae were kill with a sterile metal pin, and the treated skeletons were returned to the respective hives. The hygienic behavior was quantified as the removal of dead larvae from pupae in the cells. The percentage of uncapped larval cells and the percentage of dead larval cells were record for both bee stocks at 12 h, 24 h, and 48 h.
2.2 Hygienic behavior of honey bees towards parasitic Varroa larvae
To investigate the hygienic habits of honey bees, two colonies of equal number of bees were kept in hives at Langstroth. Four colonies were select to compare the hygienic behavior of two queen species, Apis mellifera and Carniola, towards larval nests artificially infested with mites. All mites were collect from a local colony of western honey bees from newly hatch larvae (pre-pupal or albino stage).
We select 50 pupae of worker honey bee nests that had been close the day before and open these nests at one edge with solvent-cleaned forceps. In each treated nest, a female exosmotic mite was introduce with a fine camel-hair brush. After the mite was implant, the nests were cover again with drops of warm wax and their positions were mark on a transparent sheet. In the control case, the nests of worker bees were perforate with the help of a small sterile needle and then closed again without the insertion of the varroa mite.
This was done to check whether the bees remove the honeycombs because of the presence of mites or manipulate the nests with the needle. After treatment, the honeycombs were return to the respective colonies. At intervals of three, five, and seven days, the number of larval chambers emptied by treat and control bees was measure. Three replicates were run for each test series.
2.3. Comparison of Varroa mites reproductive behavior in different hive sizes
The reproductive behavior of Varroa mites was determine based on the differences in the size of the cells of different subspecies of the Western honey bee (Carniola and Italian) on the substrate. For this purpose, they occupied two colonies, one with a Carniola queen and the other with an Italian queen. Both bees were supplement with a sucrose syrup diet. Drones did not lay eggs in each colony. The foundation was cut horizontally in two parts: 2 inches and 3 inches. Three combs were introduce in each colony and these combs were reintroduce in each colony. The width of the cells was measure with a vernier caliper to observe the size of the cells. The reproductive behavior of mites was observe in the cells of different sizes. After the data were record, an interval of one week was observe.
2.4. Comparison of Varroa mite infection rates in new and old beehives and worker beehives
Six European honey bee colonies were select to compare the infestation rates of mites in the egg nests of drones and workers. The colonies were maintain in hives at Langstroth. Each colony had a healthy queen with new and old combs. The queen laid eggs in both nests during the same period. Subsequently, the larval nests of 20 working workers and 20 drones from new and old combs were test for mite infestation. The mite infestation rates were calculate. The data were record at two-day intervals.
Statistical analysis
Quantitative data were express as mean ± standard error (S error), and all statistical data were analyze using the statistical software package SPSS (version 26). The Student’s t test was use to test for statistical significance between two groups, and one-way analysis of variance and Tukey’s post hoc statistical analysis were use to determine differences between three or more groups. The mean values of hygiene behaviors were compare with other means at the 0.05 level.
3. Results
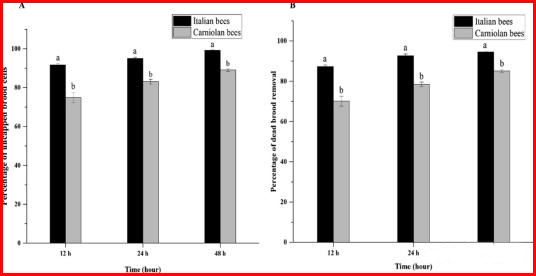
3.1. Comparison of hygienic behavior among bee species
The opening rate and removal rate of the hive were use as indicators of the level of bee hygiene behavior, and the effects of the opening rate and removal rate of the hive on the hygiene behavior of bees were study. The results (Figure 1A, b) showed that compared with the Carniola honey bee colony, the percentage of opening and removing dead hive larvae in Italian honey bees was significantly higher. The results showed that after 12 hours, the opening rate of Italian honey bees was 91.75%, significantly higher than the 74.92% of Carniola honey bees. After 24 hours, the opening rate of Italian honey bee hive pupae was significantly higher than that of Carniola honey bees, at 95.08% and 83.08% respectively. After 48 hours, Italian honey bees removed 99.25% of the dead larvae, which was still significantly higher than the 89.08% of Carniola honey bees.
The results showed that after 12 h, the removal rate of dead larvae in Apis mellifera was 87.33%, significantly higher than the 70.17% in Apis carniola. After 24 h, Apis mellifera removed significantly more dead larvae than Apis carniola (92.67% and 78.50%, respectively). After 48 h, Apis mellifera removed 94.50% of dead larvae, significantly higher than the 85.17% in Apis carniola. Overall, the opening and removal rates of dead larvae in Apis mellifera differ significantly over time, while no significant differences were observe in Apis carniola colonies after 12, 24, and 48 h.
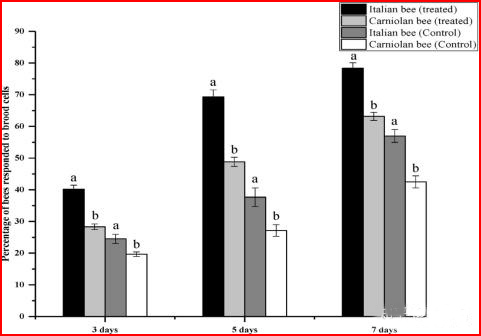
3.2. Comparison of hygienic behavior of bee species in response to the removal of artificially infested mite larvae hives
In the colonies of Apis mellifera and Carniola honey bees, significant differences were found in the hive cells artificially infest with mites on the day of examination (Figure 2). After three days, Apis mellifera and Carniola honey bees emptied 40.17% and 28.33% of the artificially infested cells in the treated colonies, respectively, while the removal rates in the control colonies were 24.50% and 19.67%, respectively. After 5 days, Apis mellifera removed 69.33% of the eggs, while Carniola removed 48.83%. The removal rates in the control group were 37.67% and 27.17%, respectively. Similarly, the percentage of infected larvae removed by Apis mellifera after an interval of 7 days was 78.33%, while that of Carniola honey bees was 63.17%. In the control group, the hatching rates in the Apis mellifera colonies and Carniola honey bees were 57.00% and 42.50%, respectively.
3.3 Comparison of Varroa mite reproductive behavior based on the size of the cells on the nest foundation
The results showed that the two types of honeycombs had significantly different cell apertures. The data were record on the cell aperture (inside) of the honeycombs. The average inner width of the Italian honeycombs was significantly different from the inner width of the Carniola honeycombs (5.005 mm and 5.276 mm, respectively) (Table 1). The inner diameter of the cells of the Italian honeycombs ranged from 5.003 to 5.006 mm, with no statistically significant difference, while the cell size of the Carniola honeycombs ranged from 5.144 to 5.457 mm (Table 1).
It is obvious that the maximum percentage of worker larvae infect with mites was found in Carniola honeycombs, while lower infection rates were record in Italian honeycombs. However, there was no significant difference in the infection of Varroa mites in Carniola and Italian honeycombs (Table 2). The infestation rate of mites in a colony may be related to the size of the hive cells. Table 2 shows that within the same colony of Carniola honeybees, larger larval cells were more susceptible to infection than smaller larval cells. The average percentage of infection in Carniola honeycombs was significantly higher than that in Italian honeycombs, 22.78% and 10.28%, respectively (Table 3). In Carniola honeycombs, the percentage of Varroa mites was significantly higher than that in Italian honeycombs (30.27% and 12.22%, respectively) (Table 3).
In the case of Apis mellifera, the cell aperture was negatively correlate with the number of mites per cell and there was no significant difference. Although there were positive and negative correlations in Carniola honey bees, there was no significant difference between the internal width of the breeding cells and the mite infestation (Table 4). Almost similarly, the internal width of the cells was negatively correlate with the number of mites per cell, while the difference between the two honey bee colonies was not significant (Table 4). There was a positive correlation between the number of Varroa mites and the number of Varroa mites in all worker bee cells, while it was highly significant in the Italian and Carniola honey bee colonies (Table 4).
Table 1 Inner cell width of worker hives of Apis mellifera and Apis carnivora (mm ± SD).
| Bee Swarm | average value | Bee Swarm | average value |
| Italian Bee | 5.048 ± 0.7919b | Carniola | 5.144 ± 0.1918 ab |
| Italian Bee | 5.034 ± 0.06024b | Carniola | 5.457 ± 0.0363 a |
| Average cell aperture | 5.005 ± 0.0464b | Average cell aperture | 5.276 ± —0.125 a |
Table 2. Percentage of worker bee larvae infected with Varroa mites in different hive sizes of the Italian and Carniola species.
| Bee Swarm | average value | Bee Swarm | average value |
| Italian Bee | 10.833 ± 0.8333b | Carniola | 20.56 ± 1.368 a |
| Italian Bee | 9.722 ± 1.5278b | Carniola | 25.00 ± 2.32 a |
Table 3 Mean percentage of the worker population infected with Varroa mites and the mean percentage of the Varroa mite population in four honey bee populations of Italian and Carniola species.
| Italian Honeycomb | Carniola honeycomb | |
| Number of capped cells | 360 | 360 |
| Average percentage of hives infected | 10.28 ± 0.86b | 22.78 ± 1.41 a |
| Average parasitism rate of Varroa mites | 12.22 ± 1.18b | 30.27 ± 1.75 a |
3.4.: Comparison of the infection rate of mites in the nests of new and old comb workers and drone larvae
The current study shows that the infection rate of mites in drone hives is higher than that in worker bee hive pupae, regardless of whether they are new or old combs (Table 5). Taking old combs as an example, there are significant differences in the percentage of drone and worker egg hives infected with Varroa mites in all groups (Table 5). In drone bee hive pupae, the maximum infection rate of mites is 43.75%, and the minimum infection rate is 26.25%. The infection rate of worker bee larvae is as high as 31.25% and as low as 15.00%.
Table 4 Correlation between (inner width of breeding hive and Varroa mite infestation), (inner width of breeding hive and number of Varroa mite infestations) and (Varroa mites and number of Varroa mite infestations) in all working hives examined in four colonies. Three hundred and sixty hives were examine in each colony.
| Bee Swarm | Relationship between the inner diameter of nest cells and Varroa mite parasitism | Inner cell width vs No. of Varroa | No. of Varroa mite vs Varroa mite infestation | |||
| r | P value | r | P value | r | P value | |
| Italian bee, Italian bee, Carniola | —0.005—0.2970.232 | 0.9850.36090.548 | —0.005—0.421—0.471 | 0.9850.6640.2004 | 10.7180.498 | 0.000*** 0.026* 0.172 |
| Carniola | —0.207 | 0.593 | —0.266 | 0.489 | 0.923 | 0.0004*** |
Table 5 Infection rate of newly combed adult female mites on drone and worker bee egg hives.
| Bee Swarm | Infection rate of Varroa mites in old nest spleen | Evaluation of new honeycomb | ||
| Drone cell infection rate (%) Average | Average infection rate of worker beehives (%) | |||
| Drone cell infection rate (%) Average | Average infection rate of worker beehives (%) | |||
| M-101 | 43.75 ± 2.63 aA | 28.75 ± 3.50b AB | 33.33 ± 3.33 a A | 18.75 ± 3.50b A |
| 4 | 26.25 ± 3.24 a B | 17.50 ± 4.12 a BC | 13.75 ± 3.24 a B | 10.00 ± 1.18 a A |
| 3 | 32.50 ± 3.66 aAB | 15.00 ± 3.27b C | 26.25 ± 3.24 aAB | 10.00 ± 2.67b A |
| 18 | 41.25 ± 4.41 a A | 27.50 ± 3.36b AB | 20.00 ± 3.78 a B | 11.25 ± 3.50 a A |
| 77 | 43.75 ± 1.83 aA | 31.25 ± 2.62b A | 35.00 ± 2.67 a A | 21.25 ± 2.27b A |
| 73 | 33.75 ± 3.24 aAB | 17.50 ± 2.50b BC | 22.50 ± 4.12 aAB | 11.25 ± 3.98 aA |
In the new hives, half of the colonies showed high parasitism rates of Varroa mites in the drone larval chambers, 33.33%, 26.25% and 35% respectively. There was no significant difference in the pupae of the drone chambers infect with Varroa mites compared with the pupae of the worker chambers. On the other hand, there was a significant difference in the awareness of the mite infection in the worker chamber pupae within the colonies (Table 5). There was no significant difference in the infection rate of the worker larvae among all the colonies.
4. Discussion
Hygienic behavior is consider a behavioral defense response by honey bee workers to remove dead bee species, parasites, and inhibit the spread of infection in the colony. In the current study, the overall data showed that A. mellifera was consistently more efficient at opening and removing dead and infected larvae compared to A. carniola. This was further support by the higher prevalence of mite infestation in A. carniola compared to A. mellifera.
Hygienic behavior varies among species, subspecies, species of honey bees, and other social insects. Rothenbuhler (1964) documented two genes responsible for hygienic behavior in honey bees, one controlling decapping and the other responsible for the removal of dead larvae. Many genetic factors are thought to be responsible for hygienic behavior in honey bees. In recent years, a comparative study of the hygienic behavior of Italian honey bees and Western honey bees has been conduct.
These results are consistent with the results of the study, which showed that Italian honey bees had a higher decapping rate and removal rate of dead larvae after 24 and 48 hours. Other existing research results have compared the hygienic behavior of different honey bee species. For example, Carniola bees showed a higher ability to remove dead larvae from the combs compared to European black bees. Adjlane and Haddad (2014) reported that after 24 hours, Tunisian bees removed 91.56% and 83.55% of dead larvae. Kamel et al.
(2003) reported that the percentage of dead larvae removed by Yemeni honey bees and Carniola bees was 72.5% and 35.6%, respectively. Balhareth et al. (2012) presented similar results, namely that the removal rate of dead larvae was significantly higher in Yemeni honey bees compare to Carniola bees during the examination period.
The results of the study (Figure 2) show that the removal rate of artificially infest hive cells was significantly higher in Italian A. melitensis colonies compared to A. carnivora colonies. Similarly, significant differences were found in the removal of pupae from artificially infested hives between Italian A. melitensis colonies with new and old queens. Shrestha et al. (2020) reported that A. melitensis workers opened and removed Varroa-infested larvae significantly faster than larvae from treated larvae (χ2 = 5.5, p = 0.017).
In contrast, there were no significant differences in the response to artificially infested mites-infested hive pupae in Africanized (χ2 = 0.34, p = 0.56) and Carnivora (χ2 = 0.25, p = 0.63) colonies after three and seven-day examinations. Aumeier (2001) showed that the removal rate of parasitoids in Africanized and Carnivora colonies was 30% to 40%, mainly due to the limited growth of the parasitoid population. Vandame (1996) determined that the removal rate of naturally infested larval chambers averaged over 40%.
These colonies showed slightly improved hygienic behavior and significantly reduced the mite infestation rate in honey bee larvae and adult bees compared to colonies with poor hygienic behavior. Due to the different odors of honey bees, they are more capable of detecting and removing artificially introduced mites from any foreign source. Further Bauer et al. (2018) report that heat cues are consider to be an important parameter that plays a key role in triggering the hygienic behavior of honey bees towards larvae and pupae infested with Varroa mites.
The results also showed that the internal width of the Italian honeycombs differed significantly from that of the Carniola honeycombs (5.005 mm and 5.276 mm, respectively). Similarly, Piccirillo and De Jong (2003) reported that Italian honeycomb cells were smaller than those of Carniola honeycombs, which were 5.16 and 5.27 mm, respectively. The internal volume of Italian honeycombs was smaller compare to Carniola honeycombs. In the context of reproductive behavior, Varroa mites prefer larger cell sizes compared to smaller cell sizes. Gonçalves (1995) determined that Varroa mites prefer larger cell sizes. Smaller cell size (4.9 mm) combs reduced Varroa infestation, while bee numbers increased in larger cell size (5.5 mm) combs (Singer et al., 2019). Oddie et al. (2019) found similar results, with a significant reduction in mite reproduction in smaller cells compared to larger cells.
(Piccirillo and De Jong, 2003) reported that the highest infestation rate of Varroa in Carniola was 24.4%, while the infestation rate in Italy was 17.7%. The nest size may be an important parameter for controlling the mite infestation. In contrast, Maggi et al. (2010) found no significant correlation between the larval nest cell aperture and the number of Varroa offspring.
Taylor et al. (2008) found no significant relationship between the effect of nest cell size on the reproductive behavior of mites, but reported an increase in infestation rate in smaller nest sizes. Berry et al. (2010) reported similar results, with a higher overall mite population in smaller nest sizes (4.9 mm ± 0.08) compared to larger nest comb sizes (5.3 mm ± 0.04). These fluctuating results from various studies provide evidence that some other parameters need to be investigate, and the nest cell size has not yet been study for Varroa population dynamics. The different methods and environmental factors of each study may be the reason for the mixed results. Smaller hive size may help reduce Varroa infestation, but may not be the key factor in achieving Varroa-free colonies. This study also showed that bee species largely influences the breeding and growth of Varroa mite colonies.
The infection rate of Varroa mites in the pupae of drone bees was higher than that in the pupae of worker bees, regardless of whether they were old or new (Table 5). Marcangeli and Damiani (2017) recorded a significant difference in the infection of Varroa mites observed in new and old combs, 13.52% ± 3.35 and 6.18% ± 2.12, respectively. Varroa mites showed a greater preference for old combs.
The incidence of infestation increased with the increase in the aperture of the ovariole ovaries of both workers and drones. The infection rate of Varroa mites in the pupae of drone bees was about 8 times higher than that of workers. Odemer (2020) reported similar results, with a significant increase in the infection of mites in the pupae of drone bees compared to the pupae of worker bees. More research experiments are necessary to better understand the attraction behavior of Varroa mites in new and old combs.
5. Conclusion
Hygienic behavior plays a vital role in the colony health of different honey bee populations. The results of this study showed that Italian honey bees had significantly higher hygienic behavior in removing needle-killed larvae and artificial Varroa mite-infested cells compared to Carniola honey bee colonies. Smaller cell apertures reduced Varroa mite infestation compared to larger cell apertures. Drone larval cells had slightly higher Varroa mite infestation rates compared to worker larval cells, regardless of whether they were new or old. Investigations of other native honey bee populations could provide a basis for hygienic bees in future breeding programs. Further research should focus on how the behavior of different honey bee races affects the survival and efficiency of the entire colony in fighting mite infestations.


One thought on “Hygienic behavior of different bees species in response to Varroa mites”
Comments are closed.